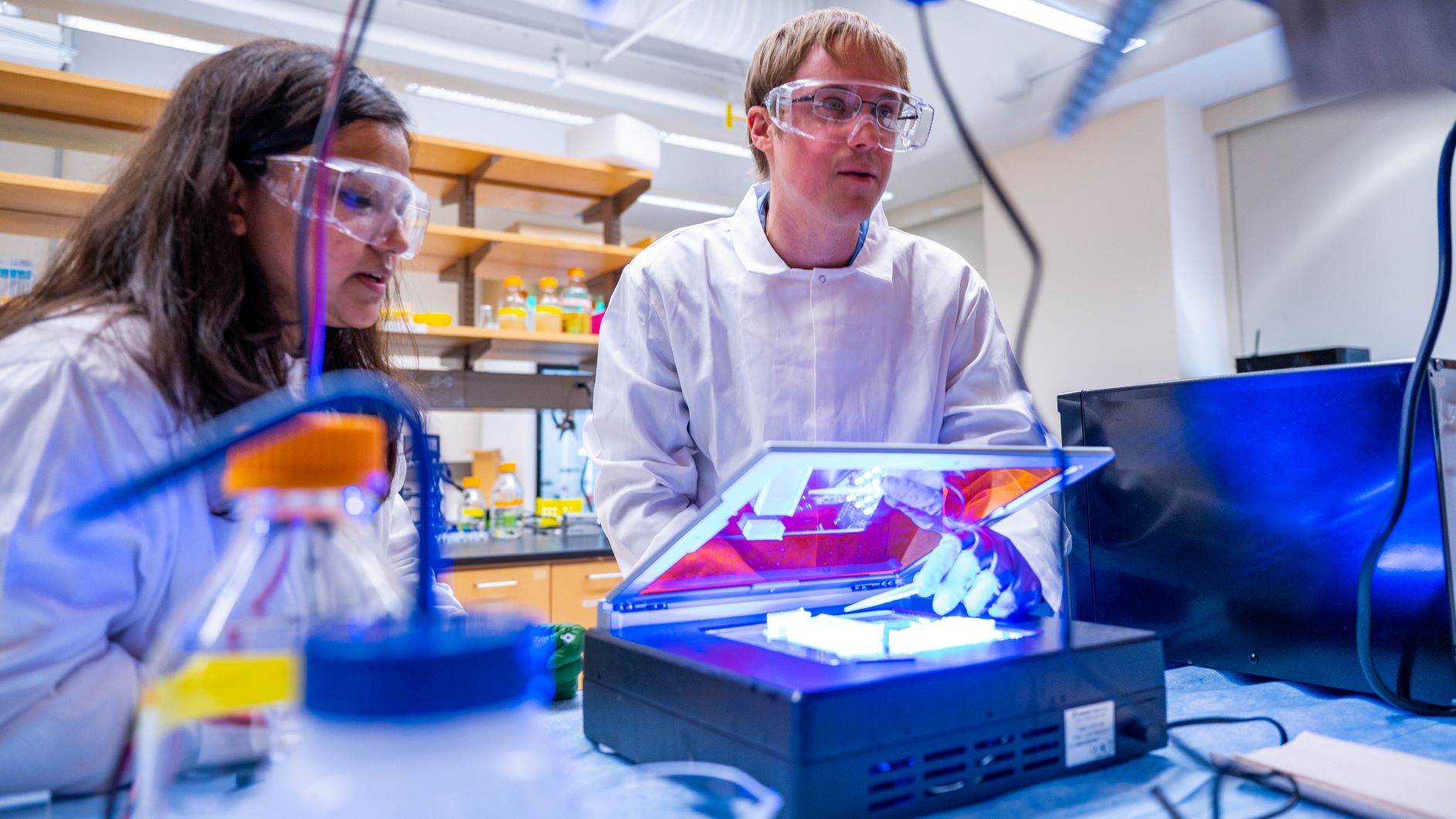
Dr. Jonathan Chekan, an Assistant Professor in the Department of Chemistry and Biochemistry, has received a new LEAP grant from the National Science Foundation for his proposal “LEAPS-MPS: Engineering and Evolution of Copper-Dependent Enzymes for Targeted Peptide Cyclization.” LEAP-MPS stands for Launching Early-Career Academic Pathways in the Mathematical and Physical Sciences, and it one of most prestigious NSF awards for early-career scientists. Learn more about Dr. Chekan’s research.
The proposal abstract, available via NSF’s award search, is below.
In this project funded by the Mathematical and Physical Sciences Directorate Launching Early-Career Academic Pathways (MPS-LEAPS) Program and managed by the Broadening Participation (CHE-BP) Program in the Division of Chemistry, Professor Chekan and his students at University of North Carolina Greensboro will study new enzyme catalysts for the crosslinking of amino acid side chains in peptides. Peptides are important molecules in multiple sectors including agriculture, research, and medicine. However, their utility is often limited by a lack of stability. To overcome this, both Nature and chemists have employed cyclization by the crosslinking of amino acid side chains as a method to improve the physical properties, stability, and bioactivity of peptides. Despite the clear benefits, introduction of these crosslinks can be difficult using traditional synthetic methods. Therefore, Professor Chekan and his students will explore two separate families of copper-dependent enzymes that are known to catalyze challenging oxidative chemistry in peptides. They will use both directed enzyme evolution and enzyme engineering approaches to improve the substrate scope and catalytic properties of these proteins with the ultimate goal of creating useful biocatalysts. Additionally, this project will serve to help bridge the transition of community college students to the University of North Carolina Greensboro by immersing them in a research focused summer program followed by a yearlong professional development series.
Professor Chekan and his students will focus on engineering copper-dependent enzymes as a platform for developing aromatic amino acid side chain crosslinking biocatalysts. To accomplish this goal, two distinct approaches will be used. First, semi-high throughput screening of copper-dependent peptide cyclases for a target reaction will be completed using cell-free protein synthesis. Promising candidates will be subject to both random and rational active site-focused mutagenesis to improve modification efficiency of the target substrate. To complement this, other copper-dependent oxidases will be developed into chimeras containing a fused peptide binding domain. This approach will localize the enzyme to the target peptide substrates of interest, allowing them to install the desired crosslinks with improved reaction rates and selectivity. Together, these two distinct strategies could not only develop new biocatalytic tools but may give basic insights into the catalytic flexibility of copper-dependent enzymes for peptide modification.